Dedicated To Helping People Survive Cancer

TOO MANY OF OUR LOVED ONES ARE DYING OF CANCER. Despite many of the advancements in healthcare, most cancers are diagnosed in the late stages of disease progression when treatments are less effective, resulting in unacceptably low survival rates.The entire Preora Healthcare team approaches each day with a clear mission: dramatically improve patient survival rates through the early detection and treatment of cancer.
Preora’s PWS Nanocytology™ platform detects early cellular changes at the nanoscale level that cause cancer, and the corresponding impact on treatments for cancer. The platform is composed of three core technologies, from sample preparation to analysis, that will increase the depth and quality of clinically relevant information. For more information, visit our Opportunities page.
Early Cancer Detection: The Key to Saving Lives Dr. Backman’s opening remarks at the Milken Institute highlighted the value of two-tiered cancer screening
Preora’s PWS Nanocytology™ Platform Includes Three Core Technologies
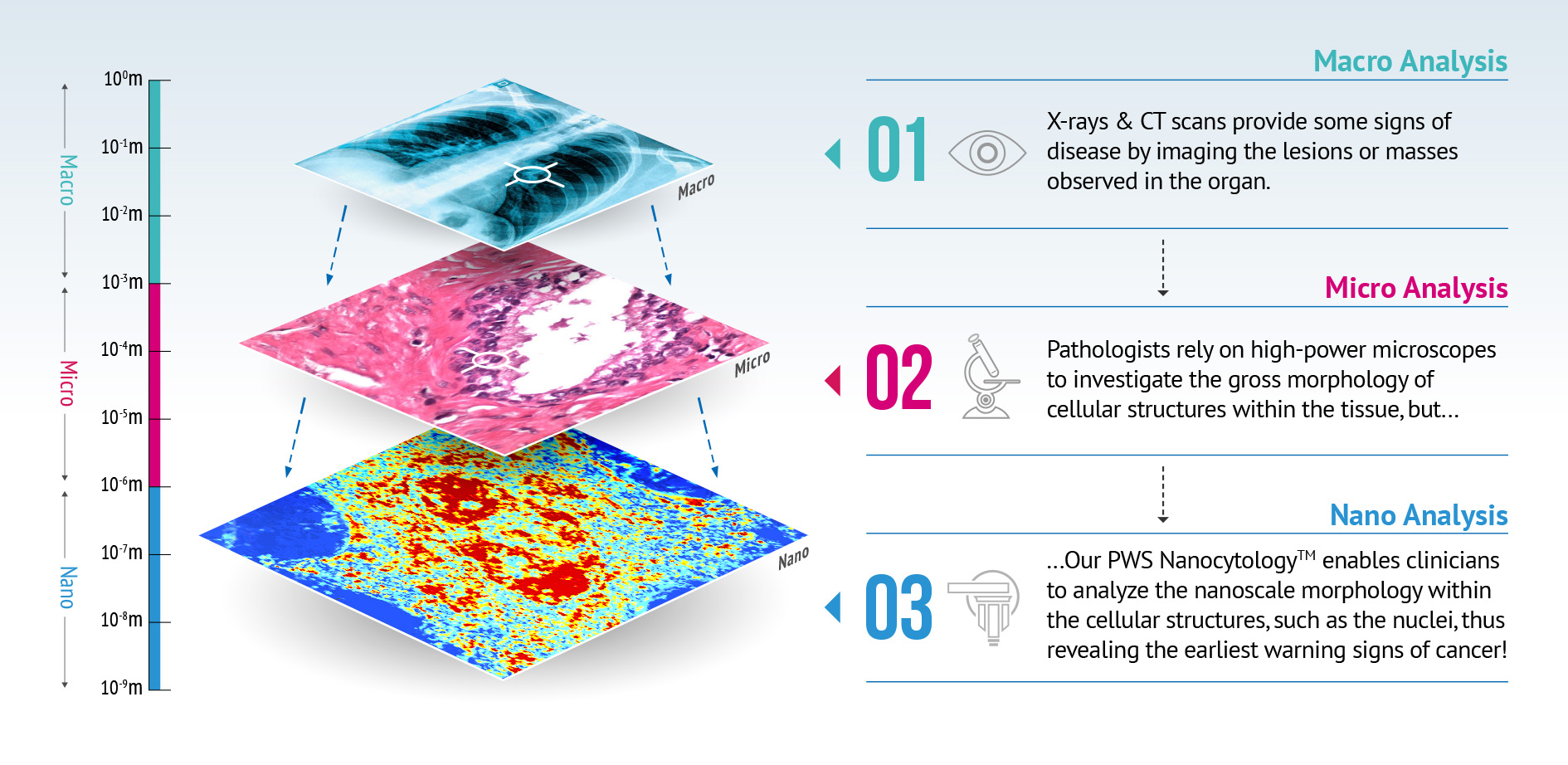
PWS Nanocytology™ detects cellular changes long before they’re evident at the “Micro” level
PWS Nanocytology™ Enables People to See Things They’ve Never Seen Before
Latest Company News
360DX.com covers agreement between Preora Healthcare and Planet Innovation
Important online trade media outlet reports on collaboration agreement, then re-posted by AACC SmartBrief (February 27, 2018). Click to view: 360Dx.com covers Preora agreement
Planet Innovation and Preora Healthcare Formalize Partnership to Bring Novel Intracellular Visualization Technology to Cancer Diagnostic Market
Planet Innovation, a leading healthtech product design and commercialization company, today announced a partnership with Preora Healthcare. The companies will collaborate in the development and commercialization of Preora’s sample-preparation technologies, and its proprietary Partial Wave Spectroscopy (PWS) Nanocytology™ platform. (Feb. 22, 2018) Click to view
Preora Expands Pipeline, Creates New Corporate Identity
Announcement of new products and name change to Preora Healthcare coincides with initiation of Series A financing round. (Oct. 24, 2017) Click to view
Preora’s Cancer Screening Platform Honored by Two Chicago Organizations
Preora has received two prestigious recognitions that acknowledge the promise of its PWS NanoctyologyTM platform to make dramatic improvements in cancer survival rates. (Sept. 21, 2017) Click to view
Preora Names Micah Litow Chief Marketing and Business Development Officer
New executive provides broad expertise commercializing medical device products. (Sept. 20, 2017) Click to view
Read More





